Patient experiences
Hair loss is a very personal issue that many women and men do not like to talk about. But why not? There is no reason to be ashamed of... | more
Pantogar® capsules

The effectiveness and good tolerability of Pantogar® capsules has been proven in comprehensive scientific studies.1-3
A meta-analysis (evaluation of several studies) of Pantogar® capsules, which comprises the results of 11 scientific studies involving a total of over 1,600 patients, proves the effectiveness of treatment with this combination of active ingredients for diffuse hair loss.
Finner, A. M. , Significant improvement of diffuse telogen effluvium with an oral fixed combination therapy a meta-analysis. Int J Trichology 3.S1 (2011): S35S50.

At a dermatology conference in 2012, dermatologist Dr. med. Andreas Finner (Berlin) presented results of a study on the use of a combination of the active ingredients cystine, thiamine, calcium panthothenate and medicinal yeast in women with diffuse hair loss. This meta-analysis comprises the results of 11 scientific studies involving a total of over 1,600 patients and proves the effectiveness of treatment with this combination of active ingredients for diffuse hair loss.
Finner AM. Nutrition and Hair Deficiencies and Supplements. Dermatol Clin, 2013. 31: p. 167-172.
In a clinical and blinded* study of 30 otherwise healthy women, Lengg and colleagues investigated the effect of a product containing a combination of the active ingredients cystine, thiamine, calcium panthothenate and medicinal yeast for the treatment of diffuse hair loss. Taking the active ingredient medication for 6 months led to a normalisation of the number of hairs that were in the growth phase (anagen hair rate) and improved the overall impression of the hair status4.
Another clinical and blinded* study by Budde and colleagues of 72 patients showed an increase in the number of hairs in the growth phase (anagen hair rate) in the group treated with a combination of cystine, thiamine, calcium panthothenate and medicinal yeast after just 4 months. Furthermore, the hair quality improved under this combination and at the same time the therapy was well tolerated5.
In a multicentre observational study by Thomas Bergner, 1,194 patients with diffuse hair loss and 642 patients with nail growth disorders were studied. After 3-6 months of use, the therapeutic effect on hair loss and nail growth was assessed as good or very good by doctors and patients in 87-90% of the cases. The therapy reduced the number of hairs falling out daily to a normal level (80-100 hairs)6.
For us, however, another confirmation of its efficacy is at least as important: Pantogar® has already impressed and convinced millions of women worldwide.
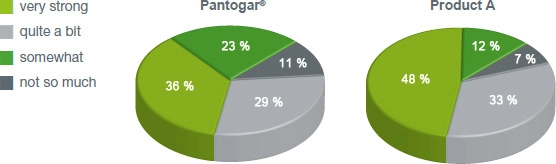
An extensive survey has shown that the treatment results of Pantogar® capsules significantly exceeded the expectations of women.**
70% of all affected women indicate that the success of the Pantogar® treatment significantly exceeded their expectations. Some of these women have told us their personal experience so that other patients can also benefit from the right treatment without unnecessary detours.
* Explanation of “blinded”: In this study, neither the doctor nor the patient knew which patients were administered the medication containing the active ingredient
** Opinion Market Research & Consulting GmbH, Germany, July 2008
1Holzegel K. Zur Behandlung des Effluviums: Ergebnisse einer Feldstudie mit Pantovigar®. Swiss Med, 1985. No. 5b. Budde J, Tronnier H, Rahlfs VW, et al. Systemische Therapie von diffusem Effluvium und Haarstrukturschäden. Hautarzt, 1993. 44:380-384.
2Petri R, Pierchalla P, Tronnier H. Die Wirksamkeit einer medikamentösen Therapie bei Haarstruckturschäden und diffusen Effluvien – vergleichende Doppelblindstudie. Switzerland. Rundschau Med. (Praxis),79(47): p. 1457-1462.
3Tsimbalenko TV, Tkachev VP, Panova OS, et al. Effect of Oral Combination of Cystine, Medicinal Yeast, and B Vitamins on Diffuse Telogen Effluvium in Apparently Healthy Women. 2008.
4Lengg, N., et al., Dietary supplement increases anagen hair rate in women with telogen effluvium: results of a double-blind, placebo-controlled trial. Therapy, 2007. 4(1): p. 59-65.
5Budde, J., et al., [Systemic therapy of diffuse effluvium and hair structure damage]. Hautarzt, 1993. 44(6): p. 380-4.
6Bergner, T., Diffuses Effluvium, Haarstrukturschäden und Nagelwachstumsstörungen erfolgreich therapiert. Der deutsche Dermatologe, 1999. 47(11): p. 881-884.